what is the percentage of nitrogen in n2o? show all calculations leading to an answer.
viii.7. Nitrogen Oxides (NOx) Emissions
NOx refers to both nitric oxide (NO) and nitrogen dioxide (NOtwo). The ecology furnishings of releasing too much NOx into the atmosphere are listed below.
- NOx is a main constituent in the formation of ground-level ozone which causes severe respiratory problems.
- Respiratory issues may result from exposure to NO2 past itself, but also of concern is NOx reacting to form airborne nitrate particles or acid aerosols which have similar effects.
- Along with sulfur oxides (SOx), NOx contributes to the formation of acrid rain and causes a wide range of environmental concerns.
- NOx can deteriorate water quality by overloading the h2o with nutrients causing an overabundance of algae.
- Atmospheric nitrogen-containing particles decrease visibility.
- NOx can react to form nitrous oxide (NorthiiO), which is a greenhouse gas, and contribute to global warming.
Coal usually contains between 0.5 and three percent nitrogen on a dry weight basis. The nitrogen plant in coal typically takes the course of aromatic structures such equally pyridines and pyrroles. The feedstock flexibility of gasification allows for a broad variation in the nitrogen content of coal.
During gasification, most of the nitrogen in the coal is converted into harmless nitrogen gas (Northwardii) which makes up a big portion of the atmosphere. Minor levels of ammonia (NH3) and hydrogen cyanide (HCN) are produced, however, and must be removed during the syngas cooling process. Since both NH3 and HCN are h2o soluble, this is a straightforward process.
In coal gasification-based processes, NOx can be formed downstream by the combustion of syngas with air in electricity-producing gas turbines. However, known methods for controlling NOx formation keep these levels to a minimum and result in NOx emissions substantially below those associated with other coal-fired electrical production technologies, as seen in the following figure.
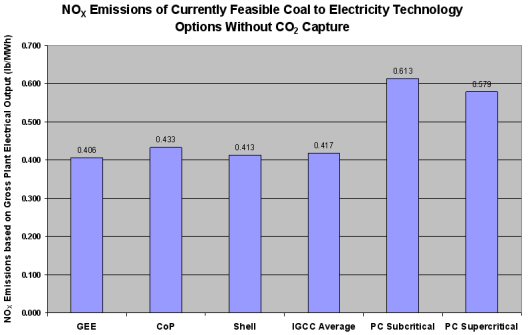
NOx Emissions Control
Although NOx emissions from operating IGCC ability plants are quite depression equally shown in a higher place, stricter regulations may require control to levels as low every bit 3 ppm in the heat recovery steam generator (HRSG) stack gas. Following is a review of both combustion-based and mail-combustion NOx control methods used for NOx emissions control.
Turbine NOx Control
Available combustion-based NOx control options for syngas-fired turbines are more than express than those bachelor for natural gas-fired turbines. The so-called Lean-Premix Technologyane, which permits the latter to reach emissions every bit low equally 9 ppm (at 15% Otwo), is not applicative to IGCC gas turbines. Differences between syngas and natural gas limerick and combustion characteristics are the source of the problem. Gasification-derived syngas differs from natural gas in terms of calorific value, gas composition, flammability characteristics, and contaminants. An oxygen-blown, entrained-flow IGCC plant will typically produce syngas with a heating value ranging from 250 to 400 Btu/ft3 (HHV basis), which is considerably lower than the one thousand Btu/ftthree for natural gas. This yields a significant catamenia charge per unit increase compared with natural gas (~14% more), resulting from the need to maintain a specified estrus input to the combustor. Furthermore, whereas the combustible composition of natural gas is primarily methane (CH4), the syngas combustible components are carbon monoxide (CO) and hydrogen (Htwo), with an H2/CO ratio mostly ranging from 0.6 to 0.82. When compared to natural gas, the H2 component of syngas exhibits a higher flame speed and broader flammability limits. The latter means that the syngas should have a stable flame at leaner conditions than natural gas, while the former indicates that the kinetics (chemical reaction speed) of H2 combustion are much quicker than that of natural gas. This very fast flame speed of the hydrogen component of the syngas prevents the employ of the lean-premix applied science. Finally, coal gasification-derived syngas will likely contain higher concentrations of H2S than natural gas, which may bear upon post-combustion NOx command technologies.
The use of a diluent to lower flame temperature, such as nitrogen or steam, is currently the preferred method for minimizing NOx generation from a syngas-fired turbine. Nitrogen is usually bachelor from the cryogenic air separation unit, so it tin conveniently be employed in the IGCC procedure. This control method can reduce NOx emissions levels from syngas-fired turbines to approximately xv ppm (at 15% O2). Full general Electric (GE) is currently targeting development of combustors to reliably achieve below 10 ppm NOx with syngas, which would exist comparable to the NOx emission levels achieved through use of the lean-premix technology on gas turbines firing natural gas.
Mail service-Combustion NOx Control
The only methods currently available to achieve single-digit NOx concentrations require handling of the flue gas to reduce the NOx to nitrogen. Selective catalytic reduction (SCR) is a fully commercial technology that has been applied to natural gas-fired turbines to minimize NOx, while the EMx (SCONOx™) process is a newer, non-ammonia based engineering science which competes with SCR.
Selective Catalytic Reduction (SCR)
SCR technology is more often than not considered as a best available add-on NOx command for stationary combustion turbines that fire natural gas or fuel oil, and is besides a candidate for utilize in IGCC. SCR selectively reduces NOx emissions past injecting NH3 into the exhaust gas upstream of a catalyst. The NOx reacts with NH3 and O2 to form N2 and H2O, primarily according to the following equations:
4NH3 + 4NO + Oii → 4N2 + 6H2O
4NHiii + 2NO2 + O2 → 3N2 + 6H2O
The goad's active surface is usually a noble metal, base of operations metal (titanium or vanadium) oxide, or a zeolite-based textile. Metallic-based catalysts are typically practical as a coating over a metal or ceramic substrate, while zeolite catalysts are typically a homogeneous material that forms both the active surface and the substrate. The geometric configuration of the catalyst body is designed for maximum surface expanse and minimum obstruction of the flue gas flow path to maximize conversion efficiency and minimize back-pressure on the turbine. The almost common configuration is a monolith, "honeycomb" design. An important gene that affects the performance of SCR is the operating temperature. Base of operations-metal catalysts take an operating temperature window for clean fuel applications of approximately 400° to 800° F. The upper range of this temperature window tin be increased using a zeolite goad to a maximum of 1,100°F. Due to the required operating temperature range for conventional SCR catalyst (600-750°F), integration into the HRSG unremarkably requires splitting of the HP evaporator (or boiler) section to conform the SCR catalyst bed and ammonia injection equipment.
An ammonia injection grid, designed to disperse the ammonia uniformly throughout the exhaust flow, is located upstream of the catalyst body. In a typical ammonia injection system, anhydrous ammonia is drawn from a storage tank and evaporated using a steam- or electric-heated vaporizer. The vapor is mixed with a pressurized carrier gas to provide both sufficient momentum through the injection nozzles and effective mixing of the ammonia with the flue gases. The carrier gas is usually compressed air or steam, and the ammonia concentration in the carrier gas is near five percent. An alternative to using anhydrous ammonia is to use aqueous ammonia. The reduced ammonia concentration in an aqueous solution reduces prophylactic concerns associated with anhydrous ammonia.
The NHiii:NOx ratio tin can exist varied to achieve the desired level of NOx reduction. Information technology takes 1 mole of ammonia to reduce one mole of NO, and two moles of ammonia to reduce one mole of NO2. College NH3:NOx ratios accomplish college NOx emission reductions, simply can result in increased unreacted ammonia being emitted into the atmosphere. This unreacted ammonia is known as ammonia slip. SCR catalysts degrade over time, which changes the quantity of NH3 skid. Catalyst life will typically range from 3 to x years depending on the specific application. IGCC applications, with frazzle gas that is relatively gratis of contaminants, should yield a significantly longer catalyst lifetime than for a conventional coal-fired awarding.
Installation of SCR in an IGCC's HRSG, for what amounts to NOx polishing, requires consideration of the ecology impacts of ammonia slip. Ammonia slip is typically limited to less than five ppm in nearly SCR applications, but may exist higher when the NOx level entering the catalyst bed is so very low. Such operation may require more excess ammonia than is typically used. While the tradeoffs between NOx and ammonia are non simple, from a qualitative perspective they are both acutely toxic; both contribute to the formation of fine particles of ammonium sulfate ((NHiv)2SO4) and ammonium nitrate (NH4NO3), acid degradation, eutrophication, and nitrogen enrichment of terrestrial soils; and both may ultimately exist converted to nitrous oxide (NorthwardtwoO), a powerful greenhouse gas. In addition, NOx (as NO2) is a chronic toxin and an essential precursor to the germination of tropospheric ozone. The contribution of NOx or ammonia emissions from a single facility to whatever of these environmental problems is primarily determined by existing levels of NOx and ammonia in the expanse and the concentration of other pollutants in the atmosphere that react with the NOx or NHthree. In terms of the range of influence or potential for long-range transport, nitric acid or organic nitrate (peroxyacetylnitrate, PAN) derived from NOx emissions, and ammonia take similar lifetimes in the atmosphere and, thus, similar potential for long-range transport. PAN and ammonium sulfate, however, are longer-lived and can spread the influence of both NOx and ammonia over a wide area.
Disposal of salt deposits and spent catalyst are as well potential environmental bug. SCR catalysts typically contain heavy metal oxides such as vanadium and/or titanium, thus creating a potential human wellness and environmental risk related to the handling and disposal of spent catalyst. Vanadium pentoxide, the most unremarkably used SCR catalyst, is on the EPA listing of Extremely Hazardous Materials. The quantity of waste associated with SCR is quite large, although the actual corporeality of agile textile in the catalyst bed is relatively small. This requires the use of licensed transport and disposal facilities and compliance with Resource Conservation and Recovery Act regulations. It is conceivable that facilities in some states may face added costs by having to dispose of these materials out of country due to a lack of licensed disposal facilities that volition handle these materials. This responsibility may not exist borne by the institute since catalyst suppliers oftentimes collect and recycle spent catalyst as part of their contract.
An additional ecology issue related to SCR is that of occupational safety. Let applicants need to exist aware of ammonia safety concerns as an effect, which in itself may mitigate the benefit of using SCR to control NOx. The EPA characterizes ammonia as an extremely hazardous substance, and vapors may grade an explosive mixture with air. Occupational Safety and Health Act regulations require that employees of facilities where ammonia is used be trained in prophylactic use of ammonia (under 29 CRF 1910.120). Facilities that handle over x,000 pounds of anhydrous ammonia or more than twenty,000 pounds of ammonia in an aqueous solution of twenty per centum ammonia or greater must prepare a Hazard Direction Plan (RMP) and implement a Take chances Management Program to preclude accidental releases. The costs for training, coming together appropriate Federal, State and local safety codes, and the preparation and approval of the RMP and Emergency Preparedness Plan must exist taken into consideration when assessing the technology. All this said, ammonia is broadly used in a variety of applications, especially agriculture, and with appropriate training can exist handled and used safely.
In that location are 2 major operational impacts resulting from the installation of an SCR organisation in the HRSG of an IGCC found. Kickoff, the pressure loss beyond the SCR catalyst bed increases the turbine back-pressure, thereby decreasing gas turbine output past approximately one-half pct. The ammonia storage and transfer equipment consumes some additional ability. 2nd, unwanted chemical reactions may negatively touch and interfere with the operation of the plant. Although IGCC fuel gas cleanup equipment efficiently removes more than than 95% of the sulfur constituent (equally H2Due south), the residual sulfur in the syngas passes to the combustion turbine where information technology is oxidized to both Then2 and So3. Ammonia slip from the SCR procedure tin can react with the SOthree forming ammonia salts such as ammonium sulfate or ammonia bisulfate. Ammonium bisulfate is a very corrosive and sticky material that tin plug downstream heat transfer equipment, reducing performance or even causing institute shutdown. The boosted back-pressure level caused by the fouling will also reduce the gas turbine output. The ammonium sulfate, if non deposited with whatever bisulfate formed, is discharged to the atmosphere equally fine particulate matter (PM2.5), since no particulate control is typically installed downstream of the HRSG. This problematic behavior represents another important departure between a natural gas-fired found and the IGCC power establish.
In order to preclude ammonia salt formation, either the ammonia slip or the And then3 must be greatly minimized. Since some ammonia slip is inevitable, IGCC suppliers recommend that a maximum sulfur oxide level of two ppm be allowed to enter the HRSG with the fuel gas. Installation of a zinc oxide or activated carbon polishing reactor, upstream of the gas turbine is one method to control the residual Thenii (with the added benefit of some added mercury control). Unfortunately, this further increases parasitic power consumption and raises the cost of the SCR installation.
EMx (SCONOx™) Oxidation/Absorption Cycle
This mail-combustion catalytic arrangement removes both NOx and CO from the gas turbine exhaust through the use of a platinum catalyst. Dissimilar SCR, information technology does non require the apply of ammonia injection, and the agile NOx removal reagent is potassium carbonate. The exhaust gases from a gas turbine flow into the reactor and react with potassium carbonate that is impregnated onto the platinum catalyst surface. The CO is oxidized to COtwo by the platinum catalyst. NO is oxidized to NOii and and so reacts with the potassium carbonate coating on the catalyst to form potassium nitrites and nitrates at the surface of the catalyst. These chemical reactions, shown below, are referred to as the "Oxidation/Assimilation Cycle."
CO + ½O2 → COii
NO + ½O2 → NOtwo
CHtwoO + O2 → CO2 + H2O
2NO2 + M2COiii → COii + KNOii + KNO3
When the carbonate becomes saturated with NOx, information technology must be regenerated. The constructive operating temperature range is 280-750°F, with 500-700°F beingness the optimum range for NOx removal. The optimum temperature range is approximately the same as that of SCR. The regeneration of the catalyst is accomplished past passing a dilute hydrogen reducing gas across the surface of the goad in the absence of oxygen. The hydrogen reacts with nitrites and nitrates to form h2o and elemental nitrogen. CO2 in the regeneration gas reacts with potassium nitrites and nitrates to reform potassium carbonate. This bike is referred to as the "regeneration cycle," as shown below.
KNO2 + KNO3 + 4H2 + CO2 → KiiCO3 + 4H2O + N2
H2o vapor and elemental nitrogen are exhausted up the stack instead of NOx, and potassium carbonate is once over again present on the surface of the catalyst, allowing the oxidation/assimilation cycle to begin again.
Considering the regeneration cycle must take place in an oxygen-free environment, the goad undergoing regeneration must be isolated from exhaust gases. This is achieved using a ready of louvers, one upstream of the section being regenerated and i downstream. During the regeneration cycle, these louvers close and a valve opens allowing regeneration gas into the section. A typical SCONOx™ organisation has five to fifteen sections of catalyst. At any given fourth dimension 80 percent of these sections are in the oxidation/absorption bike, and xx percent are in the regeneration bicycle. Considering the same numbers of sections are ever in the regeneration cycle, the production of regeneration gas gain at a abiding rate. A regeneration wheel typically is fix to concluding for iii to five minutes, so each department is in the oxidation/absorption bike for nine to fifteen minutes.
Several critical problems associated with the use of this engineering are:
- Goad is very sensitive to sulfur, including trace quantities that are typically found in IGCC frazzle gas;
- Reliability of moving parts over time is an operational and maintenance concern;
- Employ of hydrogen for regeneration could be a serious safety concern, since it is hard to contain;
- Calibration-up issues for big gas turbines;
- SCONOx™ has about twice the pressure drop of SCR; and
- The initial majuscule cost is nearly 3 times the cost of SCR, although this may come down once there are more systems in functioning.
In 1997, the EPA monitored the awarding of SCONOx™ on a natural gas-fired turbine at the Federal Cogeneration facility in Los Angeles, where it established a three.5 ppm (at 15% oxygen on a 3-hour rolling average) standard for NOx. The SCONOx™ control organisation has typically accomplished average NOx emissions of approximately 2 ppmv. This resulted in being designated every bit having achieved a LAER (everyman achievable emission rate) at iii.5 ppmv, which ready the standard for future command technology for similar facilities per Section 173(a)(2) of the Clean Air Act.
The South Coast Air Quality Direction District designated SCONOx™ every bit the Best Bachelor Control Engineering science (BACT) for natural gas-fired turbine engines. A further improvement in reductions was certified in 1998, when the EPA found that SCONOx™ had achieved a LAER of 2.v ppmv.
Post-combustion NOx reduction at IGCC Facilities in the United States
Information from permits/permit applications on recent IGCC projects includes NOx emissions rates equally shown in the accompanying effigy. Notation that not all IGCC projects, among them the very recently executed Duke Edwardsport IGCC-based ability plant and Mississippi Power's Kemper County IGCC, have been required to implement post-combustion NOx control, though others such as HECA in California are planning on doing so with SCR, which correlates with decreased NOx emissions. Edwardsport may opt to implement SCR, which would reduce its NOx emissions past approximately 2-thirds from the corporeality shown in the figure.
![NOx emission rate comparison for IGCC projects [Source: Environmental Performance of IGCC Power Plants, Steve Jenkins (CH2M HILL, Inc.) & George Booras (Electric Power Research Institute), 4th International Freiberg Conference on IGCC and XtL Technologies, May 3, 2010]](https://www.netl.doe.gov/sites/default/files/inline-images/NOx-emission-rate-IGCC.png)
[Source: Environmental Performance of IGCC Power Plants, Steve Jenkins (CH2M Colina, Inc.) & George Booras (Electric Ability Enquiry Institute), 4th International Freiberg Briefing on IGCC and XtL Technologies, May three, 2010]
Finally, it is interesting to meet the effects of implementation of SCR on NOx and other pollutant species in the context of an IGCC constitute. The following chart shows the emissions of major regulated pollutants for Duke Free energy's Edwardsport IGCC plant, both without and with SCR in operation, in comparison with the limits specified in the Class Five NSPS permit. Patently, NOx is reduced significantly with SCR and other pollutants are subtly afflicted. However, in either case, emissions of all regulated pollutants are well below the let-specified limits.
| Edwardsport IGCC Found Combustion Turbine Emissions, lb/60 minutes | |||
| Syngas-firing, 100% load (59°F), SCR Off | Syngas-firing, 100% load (59°F), SCR On | Permitted Limit | |
| NOx | 169.0 | 57.0 | 633 |
| CO | 92.5 | 93.0 | 194 |
| SO2 | 28.9 | 29.0 | 633 |
| VOC | 3.3 | 3.iii | 8.4 |
| PM/PM10/PMii.5 | 36.0 | 39.ane | 80.1 |
| HiiSO4* | iii.two | 6.4 | N/A |
1. The lean-premix combustion procedure goes by a multifariousness of names, including the Dry Low-NOx (DLN) process of General Electric, the Dry-Depression Emissions (DLE) process of Rolls-Royce/Allison, and the SoLoNOx process of CATERPILLAR/Solar Turbines. Most of the commercially available systems are guaranteed to reduce NOx emissions to the ix to 25 ppm range, depending on the manufacturer, the particular turbine model, and the application. A few manufacturers take guaranteed NOx emissions in the range of 9 ppm (eastward.g., GE). As the NOx emission level is lowered, some manufacturers accept experienced problems with combustion vibration (dynamic pressure level oscillations) and premature combustor deterioration. These technologies may result in an increase in CO and unburned hydrocarbons by as much as 50 ppm.
2. Todd, D. and Battista, R., "New Developments in LCV Syngas Combustion / IGCC Experience," General Electric technical newspaper, 2001.
Ability
- Commercial Power Production based on Gasification
- Typical IGCC Configuration
- IGCC Procedure System Sections
- IGCC Efficiency / Operation
- Syngas Limerick for IGCC
- IGCC Project Examples
- Nitrogen Oxides (NOx) Emissions
- Sulfur Oxides (SOx) Emissions
- Gasification-based Cogeneration/Coproduction
- DOE Supported R&D for Production of Ability
- Turbines Programme
- NETL Research, Development and Demonstration in Solid Oxide Fuel Cells for Power Generation from Coal

Source: https://www.netl.doe.gov/research/Coal/energy-systems/gasification/gasifipedia/nitrogen-oxides
0 Response to "what is the percentage of nitrogen in n2o? show all calculations leading to an answer."
Post a Comment